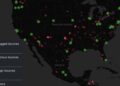
In a significant leap forward for medical diagnostics, the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance to Exo for its AI-powered ultrasound application on the Exo Iris device, specifically designed for the rapid and accurate detection of pleural effusion and consolidation/atelectasis.
This landmark approval marks a pivotal moment in the integration of artificial intelligence into point-of-care ultrasound (POCUS), promising to transform how lung conditions are diagnosed at the bedside.
Pleural effusion, a condition characterized by fluid accumulation around the lungs, can be a sign of various underlying serious health issues, including pneumonia, heart failure, and even cancer.
Traditional diagnostic methods often involve chest X-rays or CT scans, which may require patients to be transported to radiology departments, top to potential delays in diagnosis and treatment.
The newly approved Exo Iris, equipped with its advanced AI, offers an on-device, real-time solution that can detect these critical pulmonary findings within seconds. This means clinicians can obtain objective data at the patient’s bedside, eliminating the need for off-site analysis and significantly accelerating the diagnostic process. This is particularly crucial in emergency settings where every second counts.
According to Exo, this is the first FDA-cleared device of its kind, further solidifying the potential of AI to enhance diagnostic capabilities in accessible, handheld ultrasound technology. The AI algorithms, validated through rigorous clinical studies, have demonstrated “excellent” sensitivity and specificity in detecting fluid around the lungs and areas of collapsed lung.
Dr. Arun Nagdev, Exo’s VP of Clinical Affairs, emphasized the transformative impact of this technology, stating, “This innovative real-time AI provides a remarkable assist for all clinicians at the bedside, instantly and accurately detecting fluid around the lungs or areas of collapsed lung, key markers for significant infections like pneumonia or tuberculosis.” He added that it will greatly support clinicians addressing common symptoms such as shortness of breath, coughing, pain, and fatigue.
The implications for patient care are profound. Earlier and more accurate detection of pleural effusion can lead to timelier interventions, potentially improving patient outcomes and reducing the need for more invasive or delayed diagnostic procedures.
This FDA approval underscores the growing trend of AI revolutionizing medical imaging, making expert-level diagnostics more accessible and efficient across diverse healthcare settings, even without an internet connection.








![Online Scam Cases Continue to Rise Despite Crackdowns on Foreign Fraud Networks [Myanmar] Online Scam Cases Continue to Rise Despite Crackdowns on Foreign Fraud Networks [Myanmar]](https://sumtrix.com/wp-content/uploads/2025/06/30-12-120x86.jpg)